- Home
- /
- Cognitive Neurology
- /
- Pre-attentive Perceptual Organization

The human visual system organizes detected signals in a rapid, automatic, and parallel manner to enable efficient higher order processing (Healey, Booth, & Enns, 1996). We have evolved this ability to reflexively shield from threats in nature (Öhman, 1997). A UX designer can leverage this ability to craft an interface that is clear to comprehend and comfortable to use. This paper explains this evolutionary advantage by tracing its journey in the visual processing system.
Physiological Modification in the Eye
Humans are capable of inspecting different parts of the visual environment through frequent eye movements called Saccades. These rapid eye movements end with a fixation in which light from “interesting spots” in the environment is focused on the most photosensitive area of the eye, fovea (Deubel & Schneider, 1996). These ballistic movements, initiated in around 200 milliseconds, cannot be adjusted during a saccade (Healey et al., 1996; Riggs, Merton, & Morton, 1974). On the other hand, after the eyeball moves to a new spot, it takes some time to refocus and saccades that occur during a fixation allow more light into the retina (Campbell & Westheimer, 1960; Hikosaka, Takikawa, & Kawagoe, 2000)
Physiological evidence proves that specialized receptors in the eye analyze visual stimuli based on selective properties and map them to the brain (S. M. Zeki, 1976). Rods and cones present in the retina provide input to two coalesced but physically dissimilar retinal ganglion cells: the larger Type A and the smaller Type B cells (Leventhal, Rodieck, & Dreher, 1981). Moreover, the receptive fields of these cells can be classified into On-center off-surround, fields that get excited when the concentric center receives light and inhibited when the surrounding area senses light, and Off-center on-surround, fields that respond in precisely inverted style (Famiglietti & Kolb, 1976). Type A cells project their output to two magnocellular, ventral layers of the six-layered Lateral Geniculate Nucleus (LGN), and Type B cells project to the remaining four parvocellular, dorsal layers (Minkowski, 1920). The Parvo cells have smaller receptive fields that are color sensitive, and the Magno cells have larger receptive fields that are sensitive to all three cone types and also to low-contrast stimuli (Schiller & Malpeli, 1978; Wiesel & Hubel, 1966). This creates a division in the visual pathway implying a difference in receptivity (M. Livingstone & Hubel, 1988).
Neurological Tuning in the Brain
These distinctions suggest diverse contributions of the pathways connecting the LGN to the Primary Visual Cortex (V1) and their role in different aspects of vision (Hubel & Wiesel, 1972; M. Livingstone & Hubel, 1988). The cells in V1 are further tuned to extract information regarding the environment from the stream of visual data. Blobs are cells in the parvocellular pathway unique to V1 that receive both parvocellular and magnocellular inputs and are selective to color and brightness (M. S. Livingstone & Hubel, 1982, 1984). Interblob cells are areas between blobs sensitive to orientation, and layer 4B cells are sensitive to orientation and direction (M. S. Livingstone & Hubel, 1984). The information gathered in V1 is then fed to V2 which has opponent color sensitive spot cells that do not sense orientation (M. Livingstone & Hubel, 1988). The output from V1 and V2 contribute to almost 40% of visual processing (Lennie, 1998). Furthermore, higher visual areas, Middle temporal lobe is sensitive to movement and stereoscopic depth, and V4 is color selective (Dubner & Zeki, 1971; S. Zeki, 1980). Thus, the division created due to specialized retinal cells is maintained across the cortical areas using two functionally distinct yet parallel pathways: one for extracting motion, and another for obtaining form and color (M. Livingstone & Hubel, 1988). In addition, the retinotopic mapping of the visual field is preserved not only in the primary visual cortex but also in the higher cortex areas suggesting that adjacency relationships are not compromised (Schwartz, 1977). The visual pathways are connected to billions of neurons in V1 and V2 alone which enables the brain to process the entire visual field for incoming signals in parallel (Gazzaniga, Ivry, & Mangun, 2009). Therefore, V1 and V2 determine contrasting elements or features in the field of view along a number of functionally separable dimensions created by tuned neurons. Feature Integration Theory (FIT) proposes that some features are processed pre-attentively, automatically and in parallel along multiple integral dimensions in the same fixation and are combined to form a single object (A. M. Treisman & Gelade, 1980).
Pre-attentive Parallel Processing
Quantifying the term “pre-attentive” in perceptual processing has been a matter of debate among neuroscientists. However, it is commonly agreed that pre-attentive vision is achieved parallelly and simultaneously with other visual tasks (Braun & Julesz, 1998). Moreover, pre-attentive processes are resource free, and independent of human “attentive stance” because they are activated involuntarily by visual stimulation compared to attentive processes that rely not only on stimulation, but also on a specific perceptual disposition adopted by the observer (Ben-Av, Sagi, & Braun, 1992). Furthermore, the time taken to process pre-attentively is independent of the number of distractors (Braun, 1994). In their seminal work on FIT, Treisman and Gelade (1980) proposed that in the absence of prior knowledge, separable features of an object require attention for their integration. However, if features are pre-attentively grouped on applicable feature maps, attention may be directed to groups rather than individual features because this allows the brain to efficiently process more information per view (Ben-Av et al., 1992; A. Treisman & Gormican, 1988).
Perceptual Organization
The various regions recognized by the retinal mosaic are grouped together through a process called perceptual organizational (Boff, Kaufman, & Thomas, 1994). Gestalt psychologists proposed one form of perceptual organization and formulated laws based on observation to explain perceptual grouping. They believed it is the visual system’s tendency to sum discrete parts into larger wholes (Koffka, 2013; Wertheimer, 1938). Another form of perceptual organization involves analysis of textural discontinuities, an edge-based operation effortlessly performed by the visual system dividing the field of view based on texture, and perceptual grouping, a region based operation of cohesion (Julesz & Schumer, 1981; Mumford, Kosslyn, Hillger, & Herrnstein, 1987). Current theories of perceptual organization view that perceptual grouping and texture segregation are closely related and operate simultaneously or in immediate succession (Beck, Prazdny, & Rosenfeld, 1983; A. Treisman, 1982; A. Treisman & Gormican, 1988). In addition, they propose that features such as luminance, orientation, or spatial frequency are effective with respect to both grouping and segregation, on the other hand, features such as curvature and arrangement of line elements are not (Beck, Sutter, & Ivry, 1987; A. Treisman & Gormican, 1988).
Grouping Methods
The efficiency of perceptual organization is degraded when several grouping methods compete against each other in the visual space, and improved when grouping methods are employed supportively (Luna & Montoro, 2011). According to one of the grouping methods, proximity, two features are more likely to be grouped together if they are closer to each other compared to the surrounding elements, all other factors being equal (Logan, 1996; S. E. Palmer & Beck, 2007). Furthermore, two groups of features are likely to be considered separate if the distance between the closest features in the two groups is at least five times the distance between other elements within the arrangement (Gori & Spillmann, 2010). The spatial frequency model accommodates this idea by explaining how low spatial frequencies determine the perceptual grouping (Ramachandran, Ginsburg, & Anstis, 1983).
According to another grouping method, Similarity, features sharing the same visual dimension created by tuned neurons are more likely to be associated with each other. Specifically, features that are similar in elements of form (orientation and size), color, and luminosity are grouped together (Ben-Av et al., 1992; Healey et al., 1996; S. E. Palmer & Beck, 2007). Closure, a grouping method, entails closed or bounded regions to be preferred to open regions and features present within that region are grouped (Elder & Zucker, 1998). It also proposes that the perceptual system closes open gaps in contours detected through receptors to form closed regions (Boff et al., 1994; Davis & Driver, 1994). This grouping method is considered extrinsic because other elements have a role to play in forming the group (Luna & Montoro, 2011; S. E. Palmer & Beck, 2007).
Good continuation, another grouping method, predicts that features are grouped together if there is a foreseeable path from one feature to another and it can override the laws of closure (Hochberg, 1978). In fact, good continuation coupled with cues of depth can lead to closure (Ringach & Shapley, 1996). A sense of connection is established between elements that contain contours that align with each other and thus they are more likely to be grouped together (Conci et al., 2009). Palmer and Rock (1994) demonstrated that the effects of uniform connectedness are more powerful than the effects of proximity and similarity. They showed that closed regions with homogenous dimensions such as lightness, color, and texture are perceived as single units (S. Palmer & Rock, 1994). In addition, they proposed a theoretical framework in which uniform connection is considered in the initial organization of the visual field, implying that the classical grouping principles operate after checking for uniform connection (S. Palmer & Rock, 1994).
Product Review
The Tesla Model 3 is a compact all-electric sedan manufactured and sold by Tesla, Inc. It is equipped with a minimalistic dashboard with only an LCD touchscreen placed in the center of the dashboard (“Tesla Model 3,” 2019). The interfaced presented in the LCD touchscreen (Figure 1) is the target of the product review in this paper. The ergonomics of such a minimalistic system is a matter of debate in the industry, but it is out of the scope of this paper. The reason why this interface can be a rich case for pre-attentive perceptual organization is because the interface presents data with respect to every important aspect of the car. Hence, the information density in the interface is high. Furthermore, the information is organized to be accessed while the driver is driving which tests the limits of pre-attentive parallel processing. The driver of the car can use this interface primarily to access the climate control system, monitor the speed, interact with the infotainment system, and check the directions to a destination.
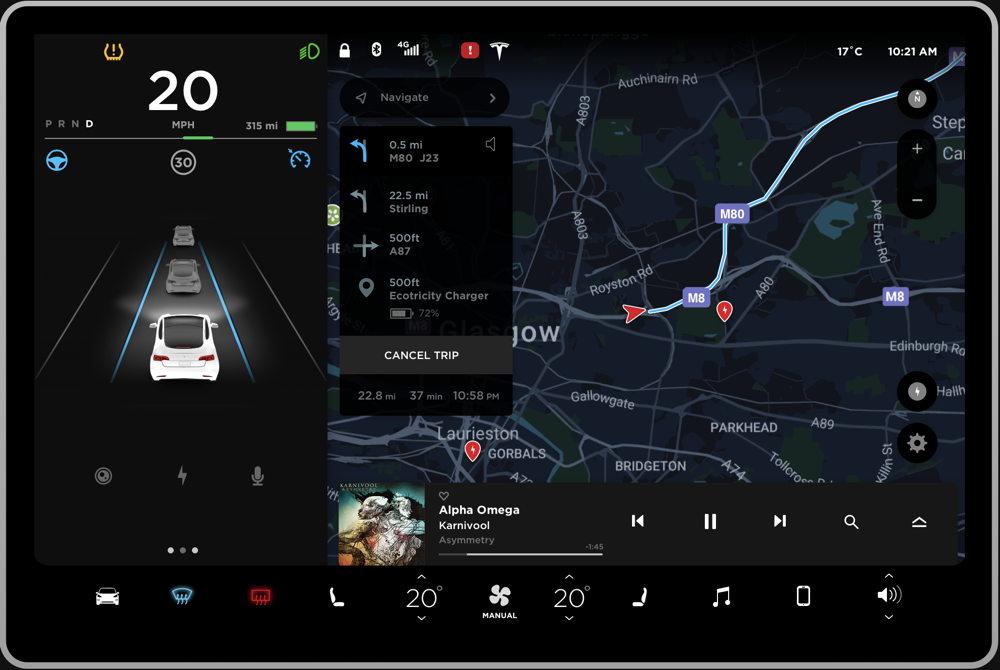
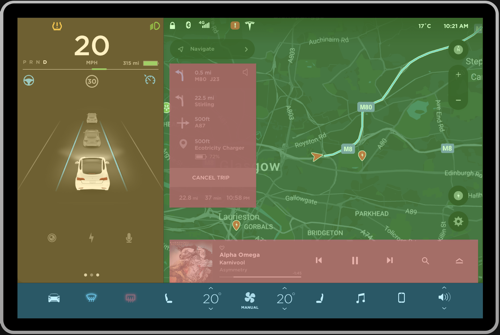
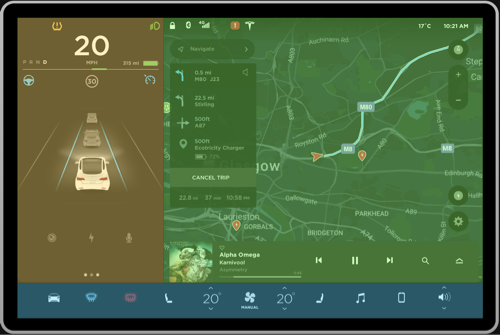
Figure 2a, 2b: Current grouping, Proposed grouping
Negative. The interface shown in Figure 1 can be pre-attentively grouped into multiple sections in a single fixation, primarily based on the principle of closure. This will make it very hard for the driver to digest the information because the bounded regions seem to fight for perceptual processing (Figure 2a). Instead, having few regions can ease the pre-attentive processing of information. To resolve this, the interface can be divided into three primary groups as shown in Figure 2b to ease higher level processing. The details of this approach will be discussed further in the review.

Positive. The region in the bottom is bounded with a rectangle that has a black background (Figure 3). This groups the elements within the closed region. The relationship is strengthened by the similarity of size among all the elements within the closed region. Furthermore, the relationship is supported by the alignment of all the elements into the same row. In addition, the driver pre-attentively finds groups within this group. The temperature indicators are followed by elements above and below them in close proximity, thus forming a group within the parent group (Highlight by yellow rectangles in Figure 3).
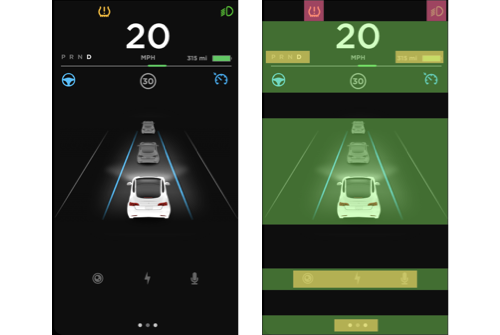
Positive. The bounded region on the left of the interface can be divided into multiple groups based on levels of horizontal alignment (Highlighted by green rectangles in Figure 4b). Some of these groups are further strengthened by the proximity of elements within the level (Highlighted by yellow rectangles in Figure 4b). Especially the text elements that indicate different states of the gearbox are very close to each other on the left side of the region compared to the elements indicating miles available in the right side of the region.
Negative. The top of the region has some floating icons (Highlighted by red squares in Figure 4b). Although these icons are vertically aligned, they do not look like a group because of the distance between them. This can be resolved by making sure the distance between the two icons is reduced.
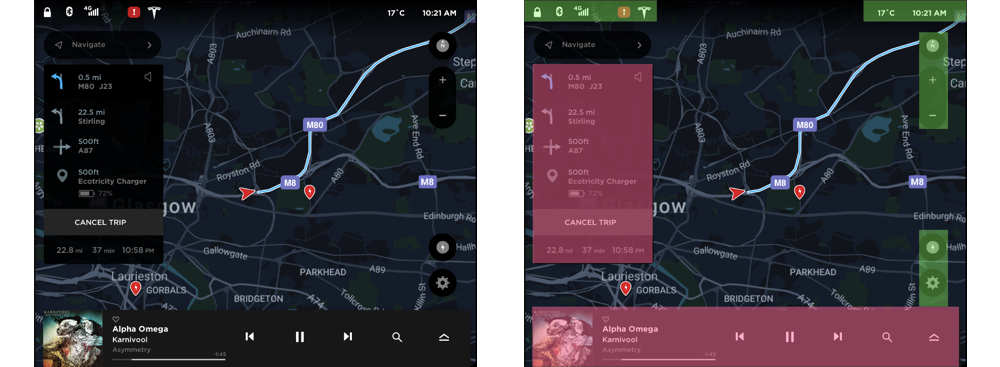
Positive. The region on the right side of the interface is automatically formed because the regions in the left and bottom are closed. This can be further divided into groups as shown in figure 5b. The groups on the right side are formed because of vertical alignment, proximity, and similarity in shape. The groups on the top are formed because of horizontal alignment and proximity (Highlighted by green rectangles in Figure 5b).
Negative. The region on the left side shows the directions to the destination (Highlighted by red rectangles in Figure 5b). This region is formed primarily because of the closed region formed by the black rectangular background. Furthermore, it is strengthened by vertical alignment, proximity, and a similarity in color. This group seems to be competing with the other main groups and can be tuned down. The region in the bottom is again primarily created due to the closed region formed by the rectangular background (Highlighted by red rectangles in Figure 5b). In addition, it is supplemented with proximity and alignment of the elements in it. This group also seems to be competing with the other main groups and can be tuned down. In both of these cases, tuning down the closed rectangular region will enable efficient organization and in turn, easier processing.
Conclusion
Humans have the ability to easily digest complex information only if it is organized into digestible chunks at first glance. Thus, designers should be equipped with the skills and knowledge required to leverage the advantage of pre-attentive perceptual organization in the human visual system into their designs. This can ensure that the end users feel comfortable using the interface.
References
Beck, J., Prazdny, K., & Rosenfeld, A. (1983). A Theory of Textural Segmentation. In J. Beck, B. Hope, & A. Rosenfeld (Eds.), Human and Machine Vision (pp. 1–38). Academic Press. https://doi.org/10.1016/B978-0-12-084320-6.50007-4
Beck, J., Sutter, A., & Ivry, R. (1987). Spatial frequency channels and perceptual grouping in texture segregation. Computer Vision, Graphics, and Image Processing, 37(2), 299–325. https://doi.org/10.1016/S0734-189X(87)80006-3
Ben-Av, M. B., Sagi, D., & Braun, J. (1992). Visual attention and perceptual grouping. Perception & Psychophysics, 52(3), 277–294. https://doi.org/10.3758/BF03209145
Boff, K. R., Kaufman, L., & Thomas, J. P. (1994). Handbook of perception and human performance. Volume 2. Cognitive processes and performance. HARRY G ARMSTRONG AEROSPACE MEDICAL RESEARCH LAB WRIGHT-PATTERSON AFB OH.
Braun, J. (1994). Visual search among items of different salience: Removal of visual attention mimics a lesion in extrastriate area V4. Journal of Neuroscience, 14(2), 554–567.
Braun, J., & Julesz, B. (1998). Withdrawing attention at little or no cost: Detection and discrimination tasks. Perception & Psychophysics, 60(1), 1–23. https://doi.org/10.3758/BF03211915
Campbell, F. W., & Westheimer, G. (1960). Dynamics of accommodation responses of the human eye. The Journal of Physiology, 151(2), 285–295. https://doi.org/10.1113/jphysiol.1960.sp006438
Conci, M., Böbel, E., Matthias, E., Keller, I., Müller, H. J., & Finke, K. (2009). Preattentive surface and contour grouping in Kanizsa figures: Evidence from parietal extinction. Neuropsychologia, 47(3), 726–732. https://doi.org/10.1016/j.neuropsychologia.2008.11.029
Davis, G., & Driver, J. (1994). Parallel detection of Kanizsa subjective figures in the human visual system. Nature; London, 371(6500), 791–793.
Deubel, H., & Schneider, W. X. (1996). Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research, 36(12), 1827–1837. https://doi.org/10.1016/0042-6989(95)00294-4
Dubner, R., & Zeki, S. M. (1971). Response properites and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Research, 35(2), 528–532. https://doi.org/10.1016/0006-8993(71)90494-X
Elder, J. H., & Zucker, S. W. (1998). Evidence for boundary-specific grouping. Vision Research, 38(1), 143–152. https://doi.org/10.1016/S0042-6989(97)00138-7
Famiglietti, E. V., & Kolb, H. (1976). Structural Basis for ON- and OFF-Center Responses in Retinal Ganglion Cells. Science, 194(4261), 193–195.
Gazzaniga, M., Ivry, R., & Mangun, G. (2009). Cognitive Neuroscience: The Biology of the Mind, Second Edition.
Gori, S., & Spillmann, L. (2010). Detection vs. grouping thresholds for elements differing in spacing, size and luminance. An alternative approach towards the psychophysics of Gestalten. Vision Research, 50(12), 1194–1202. https://doi.org/10.1016/j.visres.2010.03.022
Healey, C. G., Booth, K. S., & Enns, J. T. (1996). High-speed Visual Estimation Using Preattentive Processing. ACM Trans. Comput.-Hum. Interact., 3(2), 107–135. https://doi.org/10.1145/230562.230563
Hikosaka, O., Takikawa, Y., & Kawagoe, R. (2000). Role of the Basal Ganglia in the Control of Purposive Saccadic Eye Movements. Physiological Reviews, 80(3), 953–978. https://doi.org/10.1152/physrev.2000.80.3.953
Hochberg, J. (1978). Art and perception. Handbook of Perception, 10, 225–258.
Hubel, D. H., & Wiesel, T. N. (1972). Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. The Journal of Comparative Neurology, 146(4), 421–450. https://doi.org/10.1002/cne.901460402
Julesz, B., & Schumer, R. A. (1981). Early Visual Perception. Annual Review of Psychology, 32(1), 575–627. https://doi.org/10.1146/annurev.ps.32.020181.003043
Koffka, K. (2013). Principles Of Gestalt Psychology. Routledge. https://doi.org/10.4324/9781315009292
Lennie, P. (1998). Single Units and Visual Cortical Organization. Perception, 27(8), 889–935. https://doi.org/10.1068/p270889
Leventhal, A., Rodieck, R., & Dreher, B. (1981). Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science, 213(4512), 1139–1142. https://doi.org/10.1126/science.7268423
Livingstone, M., & Hubel, D. (1988). Segregation of Form, Color, Movement, and Depth: Anatomy, Physiology, and Perception. Science; Washington, 240(4853), 740.
Livingstone, M. S., & Hubel, D. H. (1982). Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proceedings of the National Academy of Sciences, 79(19), 6098–6101. https://doi.org/10.1073/pnas.79.19.6098
Livingstone, M. S., & Hubel, D. H. (1984). Anatomy and physiology of a color system in the primate visual cortex. Journal of Neuroscience, 4(1), 309–356. https://doi.org/10.1523/JNEUROSCI.04-01-00309.1984
Logan, G. D. (1996). The CODE theory of visual attention: An integration of space-based and object-based attention. Psychological Review, 103(4), 603–649. https://doi.org/10.1037/0033-295X.103.4.603
Luna, D., & Montoro, P. R. (2011). Interactions between Intrinsic Principles of Similarity and Proximity and Extrinsic Principle of Common Region in Visual Perception. Perception, 40(12), 1467–1477. https://doi.org/10.1068/p7086
Minkowski, M. (1920). Über den Verlauf, die Endigung und die zentrale Repräsentation von gekreuzten und ungekreuzten Sehnervenfasern bei einigen Säugetieren und beim Menschen. O. Füssli.
Mumford, D., Kosslyn, S. M., Hillger, L. A., & Herrnstein, R. J. (1987). Discriminating figure from ground: the role of edge detection and region growing. Proceedings of the National Academy of Sciences, 84(20), 7354–7358. https://doi.org/10.1073/pnas.84.20.7354
Öhman, A. (1997). As fast as the blink of an eye: Evolutionary preparedness for preattentive processing of threat. Attention and Orienting: Sensory and Motivational Processes, 165–184.
Palmer, S. E., & Beck, D. M. (2007). The repetition discrimination task: An objective method for studying perceptual grouping. Perception & Psychophysics, 69(1), 68–78. https://doi.org/10.3758/BF03194454
Palmer, S., & Rock, I. (1994). Rethinking perceptual organization: The role of uniform connectedness. Psychonomic Bulletin & Review, 1(1), 29–55. https://doi.org/10.3758/BF03200760
Ramachandran, V. S., Ginsburg, A. P., & Anstis, S. M. (1983). Low Spatial Frequencies Dominate Apparent Motion. Perception, 12(4), 457–461. https://doi.org/10.1068/p120457
Riggs, L. A., Merton, P. A., & Morton, H. B. (1974). Suppression of visual phosphenes during saccadic eye movements. Vision Research, 14(10), 997–1011. https://doi.org/10.1016/0042-6989(74)90169-2
Ringach, D. L., & Shapley, R. (1996). Spatial and Temporal Properties of Illusory Contours and Amodal Boundary Completion. Vision Research, 36(19), 3037–3050. https://doi.org/10.1016/0042-6989(96)00062-4
Schiller, P. H., & Malpeli, J. G. (1978). Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. Journal of Neurophysiology, 41(3), 788–797. https://doi.org/10.1152/jn.1978.41.3.788
Schwartz, E. L. (1977). Spatial mapping in the primate sensory projection: Analytic structure and relevance to perception. Biological Cybernetics, 25(4), 181–194. https://doi.org/10.1007/BF01885636
Tesla Model 3. (2019). In Wikipedia. Retrieved from https://en.wikipedia.org/w/index.php?title=Tesla_Model_3&oldid=886069779
Treisman, A. (1982). Perceptual grouping and attention in visual search for features and for objects. Journal of Experimental Psychology: Human Perception and Performance, 8(2), 194–214. https://doi.org/10.1037/0096-1523.8.2.194
Treisman, A., & Gormican, S. (1988). Feature analysis in early vision: Evidence from search asymmetries. Psychological Review, 95(1), 15–48. https://doi.org/10.1037/0033-295X.95.1.15
Treisman, A. M., & Gelade, G. (1980). A feature-integration theory of attention. Cognitive Psychology, 12(1), 97–136. https://doi.org/10.1016/0010-0285(80)90005-5
Wertheimer, M. (1938). Laws of organization in perceptual forms. In A source book of Gestalt psychology (pp. 71–88). London, England: Kegan Paul, Trench, Trubner & Company. https://doi.org/10.1037/11496-005
Wiesel, T. N., & Hubel, D. H. (1966). Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. Journal of Neurophysiology, 29(6), 1115–1156. https://doi.org/10.1152/jn.1966.29.6.1115
Zeki, S. (1980). The representation of colours in the cerebral cortex. Nature, 284(5755), 412. https://doi.org/10.1038/284412a0
Zeki, S. M. (1976). The Functional Organization of Projections from Striate to Prestriate Visual Cortex in the Rhesus Monkey. Cold Spring Harbor Symposia on Quantitative Biology, 40, 591–600. https://doi.org/10.1101/SQB.1976.040.01.055
